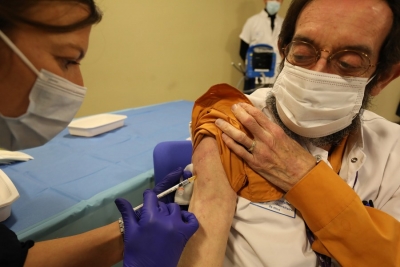
The Hague, Sep 2 : The European Medicines Agency (EMA) suggested using of two vaccines that are adapted to the Omicron variant of Covid-19, produced by Pfizer/BioNTech and Moderna.The Comirnaty Original/Omicron BA.1 (from BioNTech/Pfizer) as well as Spikevax Bivalent Original/Omicron BA.1 (from Moderna) will now be sent to European Commission for approval, after which the vaccines are able to be used by people 12 years and over who have had a an initial vaccination against the virus according to Xinhua news agency.
According to EMA research, studies have shown that the vaccines cause strong immune responses to Omicron BA.1 and the original Covid-19 strain in people who were previously vaccinationed.
The side effects that were observed with the modified vaccines were similar to those observed with those of the original, and were generally brief and not long-lasting.
“As the pandemic progresses the EU’s plan is to offer a broad variety of targeted vaccines that target different types of a disease to ensure that member states have a range of choices to satisfy their requirements when they develop the strategies for vaccination.
“This is an essential aspect in the overall plan to fight the pandemic since it is impossible to predict how the virus is likely to develop in the future, and what variants will be prevalent in the coming winter,” the EMA said.
Other vaccines that have been modified and are believed to provide better protection against variants that are not as well-known, including BA.4 and the Omicron subvariants BA.4 and BA.5 are currently being reviewed by the EMA.