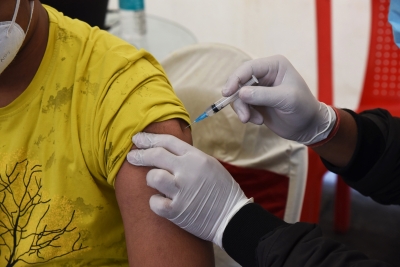
Ottawa, Aug 20 : Canada’s drug regulator announced it has authorised Pfizer-BioNTech Comirnaty Covid-19 vaccine for use as a booster dose in children aged 5 to 11 years.
Health Canada said this booster dose provides a great option to restore protection for this age group, especially for those who are at high risk of severe illness, reports Xinhua news agency.
Meanwhile the National Advisory Committee on Immunization (NACI) released national guidance for its use on Friday.
NACI recommended that children 5 to 11 years of age who have an underlying medical condition that places them at high risk of severe illness due to Covid-19, including kids who are immunocompromised, should be offered a first booster dose of the 10 mcg vaccine at least six months after completion of a primary series.
For all other children in this age group, NACI recommended the 10 mcg Comirnaty vaccine may be offered as a first booster at least six months after completion of a primary series in the context of heightened epidemiological risk.