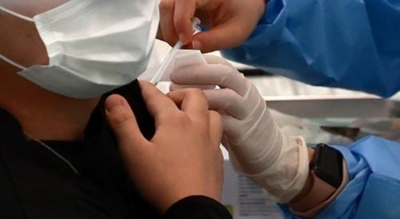
New Delhi, Dec 29 : India’s first indigenously-developed receptor binding domain (RBD) protein sub-unit Covid-19 vaccine, Corbevax, developed by Biological E Ltd, has received the Drug Controller General of India’s approval for Emergency Use Authorization (EUA).
The Department of Biotechnology and its public sector undertaking, the Biotechnology Industry Research Assistance Council (BIRAC), had supported Biological E’s Covid-19 vaccine candidate from pre-clinical stage through Phase 3 clinical studies.
“The vaccine candidate was provided financial support under Covid-19 Research Consortium, through the National Biopharma Mission, for pre-clinical toxicology studies.Later, support was provided under Mission Covid Suraksha for clinical development.
Corbevax is a two-dose vaccine administered intra-muscularly and can be stored at 2 degrees Celsius to 8 degrees Celsius,” a Science and Technology Ministry release said on Wednesday.
The recombinant protein sub-unit vaccine developed from the RB) of the spike protein on the viral surface is adjuvanted with Dynavax’s CpG 1018 and alum.
Comprehensive Phase 3 clinical trials involving more than 3,000 subjects between the ages of 18 and 80 at 33 study sites across India, demonstrated the vaccine to be safe, well tolerated and highly immunogenic.
The Translational Health Science and Technology Institute (THSTI), an autonomous institute under the DBT, provided key immunogenicity data for the Phase 2/3 studies.
“The EUA to Corbevax is yet another example of a successful academia-industry collaboration.This vaccine will sharpen the country’s efforts in ending the pandemic.
The development of indigenous vaccines to fight the pandemic will also inspire the country’s scientists and manufacturers to resolve the problems of the country,” Secretary, Biotechnology, Dr Rajesh Gokhale said.
Biological E Ltd Managing Director Mahima Datla said: “Prime Minister Narendra Modi’s vision and the advance commitments we received towards Corbevax were instrumental in our ability to scale-up and manufacture at such huge capacities.While Covid Suraksha Programme’s endeavour to accelerate vaccine development played a crucial role in the initial development, the mechanism that was setup with the support of Department of Biotechnology and DBT-BIRA) allowed us to scale up to a capacity of about 1.2 billion doses per annum making the dream of accessibility – affordability and supply – a reality.”
The Department of Biotechnology, under the Science and Technology Ministry, promotes and accelerates the development of biotechnology in India, including growth and application of biotechnology in the areas of agriculture, healthcare, animal sciences, environment, and industry.
The BIRAC is a not for profit Section 8, Schedule B, public sector enterprise, set up by the DBT as an interface agency to strengthen and empower the emerging Biotech enterprise to undertake strategic research and innovation, addressing nationally relevant product development needs.
Biological E, a Hyderabad-based pharmaceuticals & biologics company founded in 1953, is the first private sector biological products company in India and the first pharmaceutical company in southern India.Developing, manufacturing, and supplying vaccines and therapeutics, tt supplies its vaccines to over 100 countries and its therapeutic products are sold in India and the US.BE currently has eight WHO-prequalified vaccines in its portfolio.
niv/vd
#Covid #vaccine #Corbevax #Drug #Controllers #Emergency #Telugu #TeluguStop #Hyderabad #Delhi #Rajesh #Delhi #New Delhi #Covid-19 #Narendra
.